Terms of Reference
Aotearoa New Zealand All Cardiology Services Quality Improvement (ANZACS-QI) Data Governance Group document.

ANZACS-QI Programme Methodology: How Cardiology Data is Collected, Protected and Used for Quality Improvement
ANZACS-QI Programme Methodology: How Cardiology Data is Collected, Protected and Used for Quality Improvement.
ANZACS-QI is a secure, web-based cardiology clinical registry that captures data on all cardiac events and cardiology services in Aotearoa New Zealand. This information supports quality improvement, equity, and cardiovascular research, helping ensure that patients nationwide receive evidence-based treatment.
Our methodology includes a rigorous national auditing process. Dedicated coordinators regularly check the accuracy of data collection at hospitals across New Zealand. This ensures that registry data is reliable, enabling clinicians, researchers, and policymakers to:
• Monitor and improve cardiac care
• Develop clinical guidelines
• Publish high-quality research
• Track outcomes and inequities
The programme is reviewed and approved annually by the New Zealand Health and Disabilities Ethics Committee (MEC07/19/EXP), ensuring strong governance and patient protection.

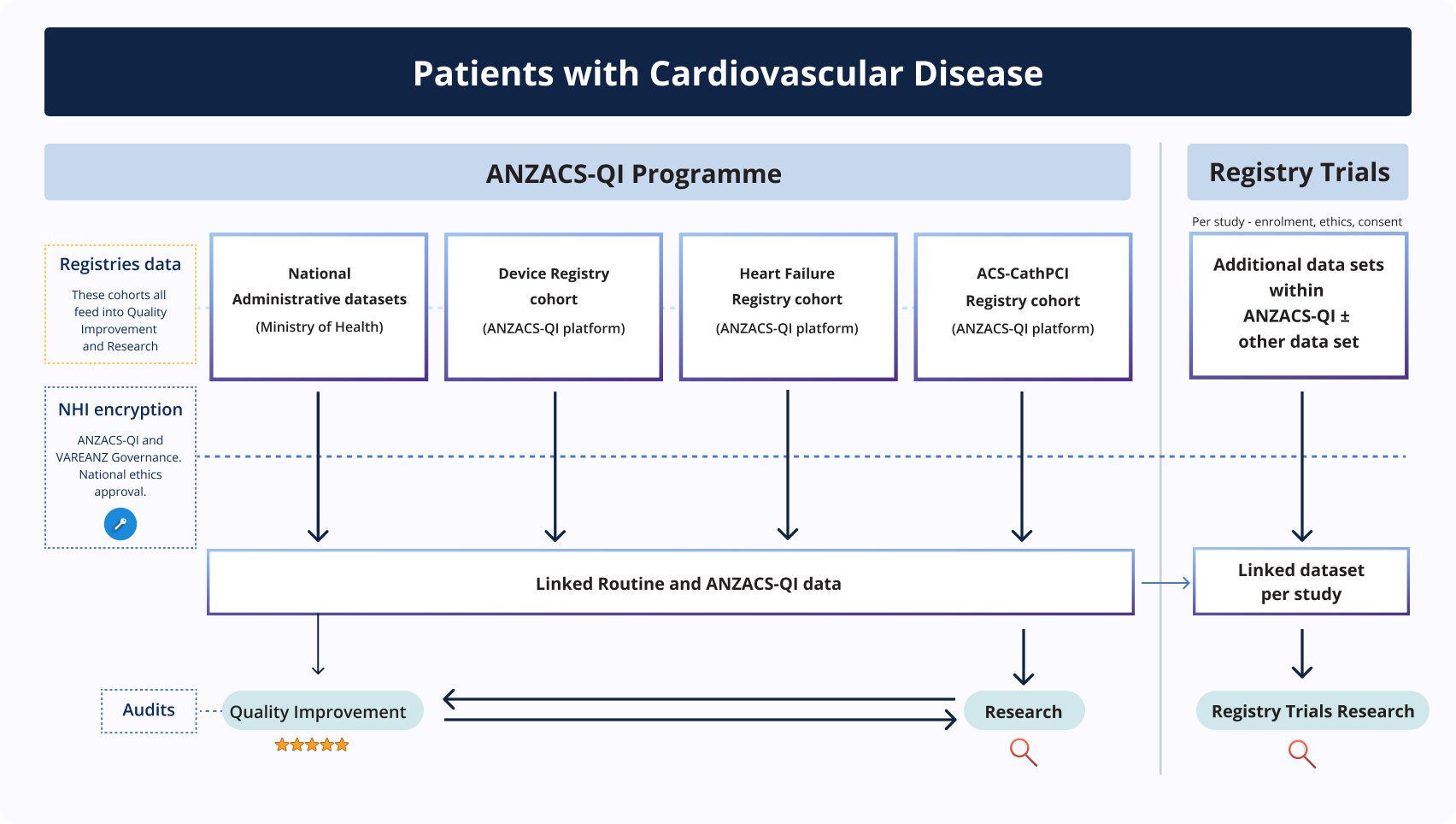
Patients are entered into the ANZACS-QI registry when they are admitted to hospital with a cardiological condition.

The clinical information recorded is available for the clinician providing patient care, to improve patient care quality.

A de-identified copy of the registry information is linked with routine information that is captured by the Ministry of Health and is used for quality improvement to inform national research.

The ANZACS-QI and VAREANZ Data Governance Groups ensure that patient data housed within the registry is gathered, managed and used appropriately to ensure patient privacy.







ANZACS-QI's distinctive approach includes a rigorous data auditing process, with national coordinators regularly assessing the accuracy of data collection at hospitals nationwide. This ensures the robustness and reliability of our data, which forms the basis of clinician recommendations and publications aimed at enhancing patient care and outcomes.
This programme has been approved by the NZ Health and Disabilities Ethics committee ((MEC07/19/EXP) with annual re-approval).
Patients are entered into the ANZACS-QI registry when they are admitted to hospital with a cardiological condition.
The clinical information recorded is available for the clinician providing patient care, to improve patient care quality.
A de-identified copy of the registry information is linked with routine information that is captured by the Ministry of Health and is used for quality improvement to inform national research.
The ANZACS-QI and VAREANZ Data Governance Groups ensure that patient data housed within the registry is gathered, managed and used appropriately to ensure patient privacy.
If you are a Health Professional and would like to gain access to ANZACS-QI data for a clinical trial, please read the DAP Data Access & Publishing Guide and complete the Data Access Proposal Request Form and send it to anzacsqi@nihi.auckland.ac.nz.
ANZACS-QI have set these indicators, which have been endorsed by the National Cardiac Network and the Ministry of Health. They are nationwide key performance indicators (KPIs) for the purpose of quality improvement of cardiac service delivery.
For a complete and detailed description of these indicators, please see
ANZACS-QI National Indicators and Targets May 2024








ANZACS-QI have set these indicators, which have been endorsed by the National Cardiac Network and the Ministry of Health. They are nationwide key performance indicators (KPIs) for the purpose of quality improvement of cardiac service delivery.
For a complete and detailed description of these indicators, please see ANZACS-QI National Indicators and Targets May 2024








